Table of Contents
Some time between now and the end of Spring 2021—some doctors expect it as early as April—Qwo should be hitting a plastic surgeon’s or derm’s office near you. What is it? What does it do? And what’s in it for you? Here’s answering your every Q on Qwo just in time for its Spring 2021 cellulite-busting debut.
Contents
1. What is Qwo? 2. What is cellulite? 3. What are fibrous septae? 4. What is Qwo's active ingredient? 5. How effective is Qwo? 6. When was Qwo FDA approved? 7. Is Qwo painful? 8. Does Qwo require downtime? 9. Am I a candidate for Qwo?
10. Does work for all skin tones? 11. Difference between Qwo and Cellfina 12. What is treatment like? 13. How many Qwo treatments will I need? 14. When will I see results of Qwo? 15. Can Qwo be used on the thighs? 16. Can men be treated with Qwo? 17. Common side effects of Qwo 18. What does "Qwo" mean?
Qwo is the World’s First-Ever FDA-Approved Injectable for Moderate to Severe Cellulite in the Buttocks of Adult Women
According to FDA-approved patient information released by the company, “QWO is a prescription medicine used for the treatment of moderate to severe cellulite in the buttocks of adult women.”
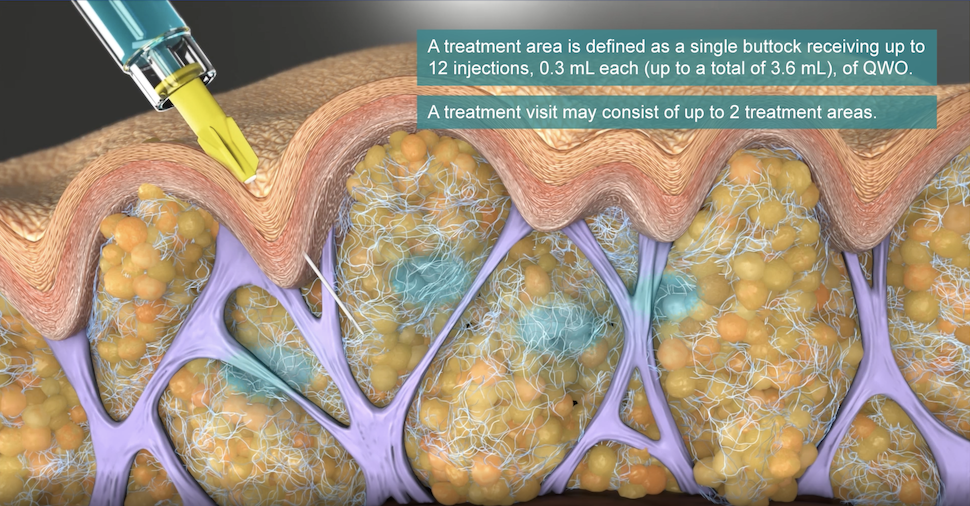
Cellulite is Fat Partitioned Off by Fibrous Septae
Up to 90% of women, and some men, are affected by cellulite. Cellulite occurs most often on the buttocks, legs, and thighs. It is believed to be caused not merely by the presence of fat, but by something within that fat—fibrous bands of strong tissue that connect the dermis to the muscle, but only at certain points. As these fibrous bands tether the skin down, the fat is also pushing (herniating) upward.
Because the skin is only tethered in so many places, divots and dimples appear on the skin surface above the fibrous connective bands, giving that unwanted orange peel, cottage cheese, or mattress-like alteration of the skin surface.
The etiology (cause) of cellulite isn’t entirely known, but there are multiple theories about it—hormonal, genetic, inflammatory, and vascular.
According to the most recent science, there are three components to cellulite: Simplistically stated, the expansion of subcutaneous fat causes the hills, the fibrous septae pulling downward cause the valleys or dimples, and the looseness of the dermis allows both of those to occur. It is also postulated that as fibrous septae get wider, they too contribute to the fat herniating upward.
Fibrous Septae are Comprised of Collagen Types I and III
The bands, called fibrous septae, are composed of collagen Types I and III. So while many cosmetic procedures, like those so often used on the face, aim to replenish healthy collagen as a key component of a youthful appearance, when it comes to cellulite, most treatments aim to destroy certain collagen to allow the skin’s surface to be more uniform and of level contour, free of the dimples and divots it contributes to.
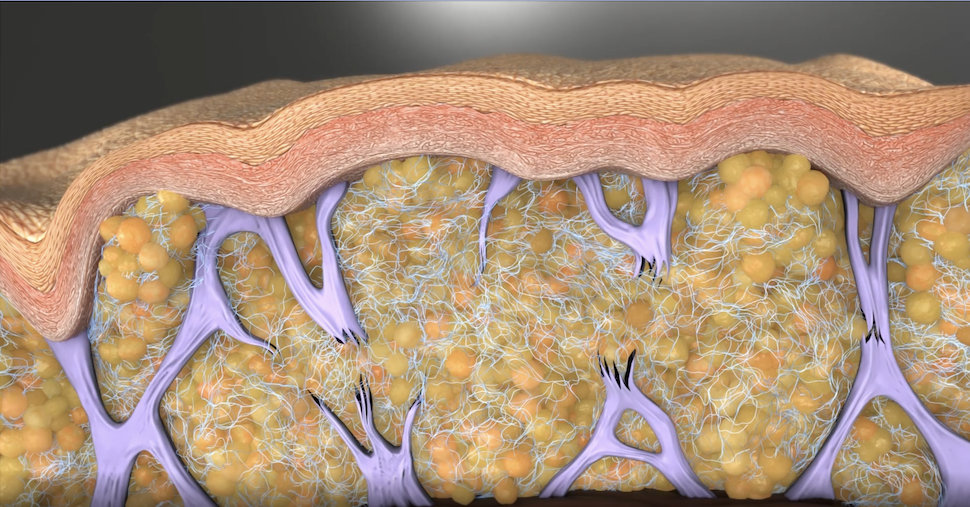
Qwo’s Active Ingredient, Collagenase Clostridium Histolyticum (CCH), is an Enzyme That Breaks Down Collagen Types I and III
Qwo’s active ingredient is “collagenase clostridium histolyticum,” or CCH. Colaganese is an enzyme that helps break down Types I and III collagen. Over time, by breaking down the collagen that makes up the fibrous septae bands causing the dimples and divots in cellulite, the skin surface and contour can be markedly improved.
Qwo Isn’t the First FDA Approved Use of CCH in an Injectable
Xiaflex is FDA approved for the treatment of Dupuytren’s contracture of the hand, and Peyronie’s disease in the penis. QWO is a different form of that same enzyme formulation, and cross-purposing the two formulations is a no. Xiaflex is also an Endo product.
To Test and Launch Qwo, Endo Ran the Largest Clinical Trials Ever Conducted on Cellulite
Endo’s double-blind placebo-controlled clinical trials involved 843 women with moderate to severe cellulite on both buttocks. Patients received either a placebo or the real deal, and ranged in age from 18 to 78-years-old, had BMIs of anywhere from 18 to 67, and skin tones that fit all classes of the Fitzpatrick skin type scale—from pale white, to dark brown or black skin. Seventy-eight percent (78%) of clinical trial participants were White, 22% had skin of color.
50.55% of Clinical Trial Participants Were “Satisfied” or “Very Satisfied” With Qwo Treatment
According to an Endo summary review of clinical studies for providers, the trials conducted resulted in patient satisfaction levels as follows, when they were asked about it on Day 71:
— 54.3% of 210 patients in a study were “satisfied” or “very satisfied” with treatment.
— 46.8% of 214 patients in another study were “satisfied” or “very satisfied” with treatment.
“Day 71” is three-weeks (21 days) after their third and final Qwo treatment session was administered, and is the same time-frame as that used for the reporting of all side effects and other results.
Patients who received Qwo were 2.56 times as likely to be “satisfied” or “very satisfied” as those who had been injected with a placebo.
Qwo was Given FDA Approval on 6 July 2020 (at 4:16:56 P.M.)
After years of R&D and trials, Endo submitted Qwo for FDA approval on 6 September 2019. It was approved in an FDA letter signed 6 July 2020. “We have approved your BLA [biologics license application] for Qwo (collagenase clostridium histolyticum-aaes) effective this date. You are hereby authorized to introduce or deliver for introduction into interstate commerce, Qwo under your existing Department of Health and Human Services U.S. License No. 2136. Qwo is indicated for the treatment of moderate to severe cellulite in the buttocks of adult women.” – Director, Division of Dermatology and Dentistry, Center for Drug Evaluation and Research
Intrigued by Qwo? Here’s What You Need to Know

Qwo Isn’t That Painful
The buttocks are home to plenty of fatty tissue, making Qwo injections far less painful than neuromodulator or dermal filler injections to the face for instance. In fact, at one practice, most Qwo patients rated their level of pain from the procedure a 0 or a 1 on a 0 – 10 scale.
The post procedural bruising is very pronounced but is also not very painful.
No Patient Downtime is Required
No recovery or downtime is required for most patients after Qwo. Tenderness and mild swelling should be expected, but most patients are able to immediately return to their social or work activities after treatment. No patient downtime was required in clinical trials.
The Ideal Candidate Has Good Skin Elasticity and Moderate to Severe Dimpled Cellulite
Qwo doesn’t resolve thin skin, poor muscle tone, or skin laxity, which are factors that contribute to the appearance of cellulite. The ideal patient has good skin elasticity but moderate to severe dimple-like cellulite. Approximately 34% of trial participants were overweight, and 48% were obese. It isn’t weight that classifies a poor candidate for Qwo, but skin health and the type of cellulite. “QWO is indicated for cellulite—not laxity,” says the company. “Patients with well-defined dimples and some laxity can still be good candidates for QWO, but the treatment may only improve cellulite dimples.” (Qwo is a prescription medication. Only a licensed M.D. can prescribe it.)
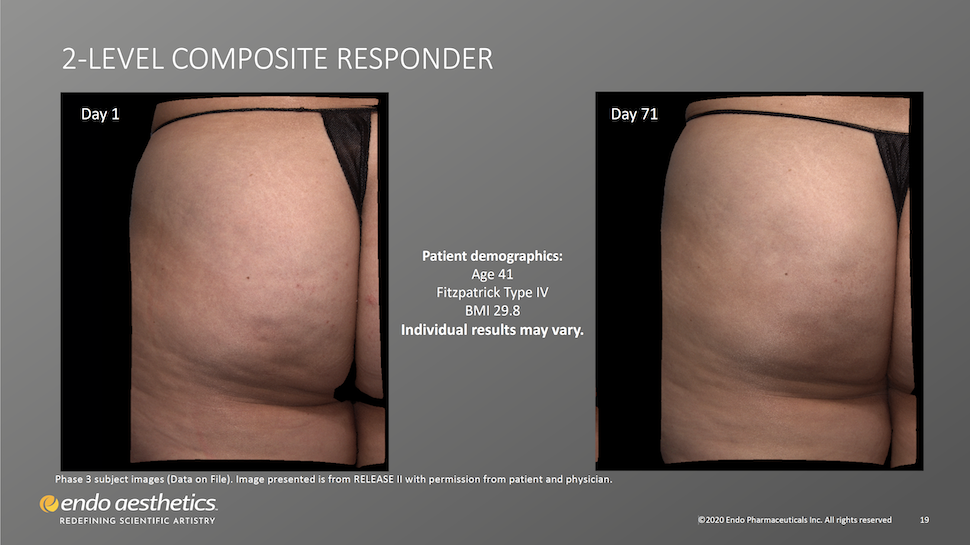
Qwo Works for Patients With Skin of Color
Twenty-two percent (22%) of patients treated with Qwo in clinical trials were non-White. 31.8% of trial participants receiving Qwo had pale white/fair skin, 28.15% had darker white skin and 40.1% had light brown, brown, or dark brown skin. These patients were satisfactorily treated with Qwo.
Qwo Does With Natural Enzymes What Cellfina Does With an 18-Gauge Blade
If you’ve given thought to treating cellulite, you’ve likely heard of—and possibly even tried—Cellfina. It received FDA approval in 2014 as a minimally invasive procedure clinically proven to improve the appearance of cellulite for at least three years—the longest such FDA clearance at the time.
A proprietary vacuum guidance system is used in Cellfina to lift the buttock tissue and subcutaneously anesthetize treatment areas before an 18-gauge reciprocating microblade is inserted to release the fibrous septae beneath dimples, releasing the bands at multiple depths.
Qwo, while not compared to Cellfina in official literature, operates much to the same end, using enzymes to do what has previously only been done with a blade (and plenty of bruising), or a laser.
Qwo injections cause an enzymatic degradation and release of the fibrous septae over time, while Cellfina takes a mechanical approach.
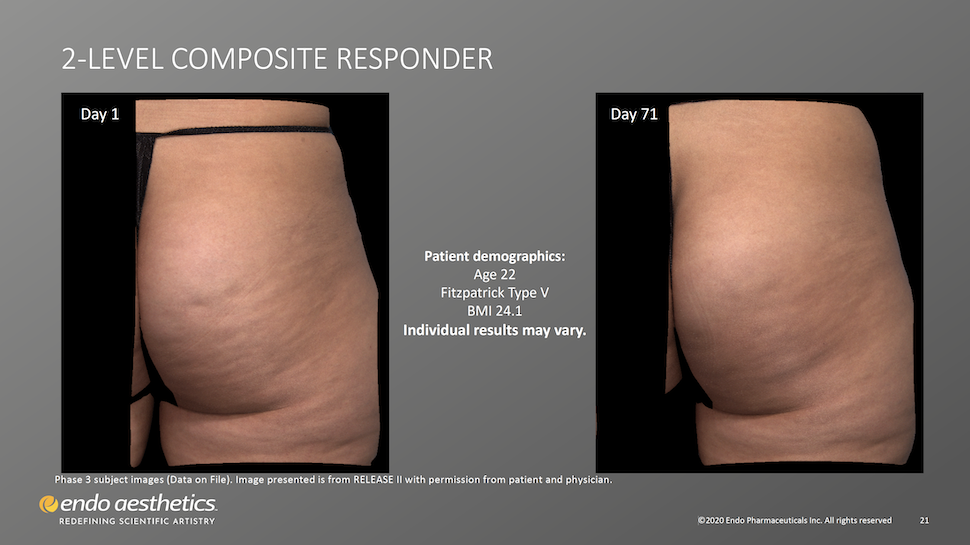
What to Expect in Treatment: Photos, Sharpie Circles, Lying Prone. And Virtually Painless Pokes.
- A Qwo provider near you (an Endo/Qwo-licensed dermatologist or plastic surgeon) will take photos of your buttocks.
- You’ll stand with the butt muscles relaxed, as your provider marks non-overlapping circles around those dimples that are well defined and evident while the gluteus maximus muscles are relaxed.
- You’ll then lay prone (face down) on the treatment table.
- Provider will inject 0.3 ml of Qwo into the center of each marked dimple three times: one injection of 0.1 ml of Qwo goes perpendicular to the skin surface (vertically straight down), the needle is then partially withdrawn and the second injection is done at about a 45 degree angle pointing toward the head, and the last injection of 0.1 ml is done at a about a 45 degree angle pointing toward the feet.
- Provider moves to the next circled dimple. Up to 24 such cellulite dimples can be treated per visit; 12 per cheek per treatment.
- When all designated cellulite dimples have been treated, you’ll remain lying down for about 5 minutes.
- You can return home immediately afterwards with no downtime but expect some discomfort, obvious bruising, and mild pain at the injection sites.
Allergies and Active Infections are Among the Contraindications
Qwo is a prescription medication that is injected into the buttocks during an in-office visit. Only a licensed medical doctor can prescribe it to a patient.
Patients who shouldn’t receive treatment with Qwo include persons who:
- Are allergic to collagenase
- Are allergic to any of the other other ingredients in Qwo
- Have an active infection in the intended treatment area
- Have certain bleeding and coagulation disorders
- Are pregnant, actively nursing or planning to nurse
- Have tattoos within 2 cm of treatment areas
3 Treatment Sessions are Done 21 Days Apart
QWO is administered in a series of three treatments spaced 21 days apart. At each treatment, both buttocks can be injected, for a maximum of up to 12 injections per buttock and 24 injections total. Treatment is done on “Day 1,” Day 22, and Day 43—all at three-week, 21 day, intervals.
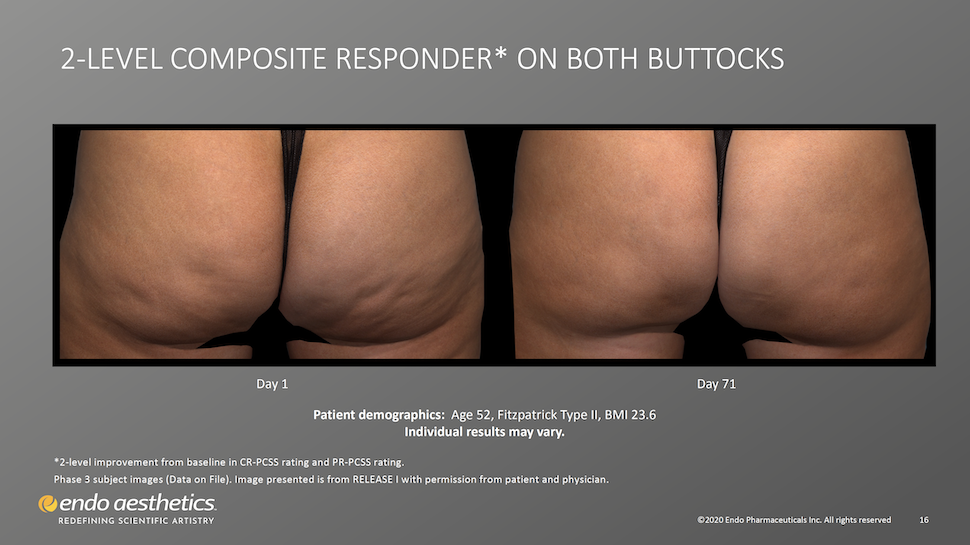
Visible Results Appear as Early as 28 Days After Treatment
Patients should expect to see a visible improvement in cellulite dimpling in as little as three weeks after injections. Qwo’s results are considered to be permanent, however an ongoing study will look at 5-year efficacy and safety. Qwo will not prevent further cellulite dimples from forming as it only targets the fibrous septae causing existing dimples.
Qwo Isn’t a No for the Legs & Thighs
Qwo is thus far only indicated for the treatment of moderate to severe cellulite in the buttocks of adult women. But if you’re hoping to have treatment elsewhere, you’ll be happy to know it’s probably a go and that it is worth asking your doctor about.
Here’s everything we know about Qwo when it comes to leg, arm and thigh treatments.
1 | Off-label use of Qwo on the thighs and arms “is possible” says Dr. Whitney Bowe
Responding to patient excitement about Qwo’s 6 July 2020 FDA approval, renowned New York-based dermatologist, Dr. Whitney Bowe announced, “Qwo was just FDA approved for moderate to severe cellulite in the buttock of women. So that’s what they got FDA approval for. Will we end up using it off label, for the back of the thighs, the sides of the thighs, maybe even the arms? It’s possible. We do a lot of procedures off label in dermatology.”
“That’s something dermatologists are used to all the time. So if you have cellulite on your lateral thigh and you read this press release about Qwo and they talk about the buttock and how it’s only FDA approved for the buttock, relax. It will probably end up being something we can apply to those other areas as well if it ends up being something that you’re interested in.”
2 | Some plastic surgeons are hopeful but uncertain about Qwo’s use in other areas
California board-certified dermatologist and cosmetic surgeon, Dr. Timothy Jochen, says Qwo can be used for the legs and buttocks of adult women. “Actually, it’s for the buttocks. But you can also use it in the legs. Hopefully.”
FDA approved patient information for Qwo says “Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information, talk to your healthcare provider.”
3 | Qwo should do “very well” in other areas of the body
For some doctors, the use of Qwo on the thighs seems to be a given. Dr. Bruce Katz, Director of JUVA Skin & Laser Center in New York participated in the clinical trials that lead to Qwo’s FDA approval. Asked by a potential patient during a virtual event about treating cellulite in the arms with Qwo, Dr. Katz said Qwo hasn’t yet been evaluated on the arms, but that the cellulite seen on the arms is typically like that seen on the thighs, so in treating it providers would use a particular technique piloted in clinical trials that involves both deep and shallow injections in five different directions of the dimple—as opposed to one deep injection and two directional shallow ones as is done on the buttocks. “And it should do very well,” he says.
4 | Clinical trials are ongoing on Qwo use in the thighs
San Diego board-certified dermatologist and plastic surgeon Dr. Melanie Palm, who served as a clinical investigator for Qwo, says “It’s actually approved for women for the buttock region,” but there are investigations underway “and we’ve been involved in some clinical trials for Qwo, looking at other areas including the thighs of women.”
5 | Endo is still researching Qwo’s use on the thighs
Endo’s VP of Sales & Marketing, Rob Catlin, spoke to this on December 1st, 2020, in episode 12 of “The Technology of Beauty Podcast” with host Dr. Grant Stevens.
Here’s what was said:
DR. GRANT STEVENS: “You mentioned the buttocks. Did you do studies also on the thighs? Either posterior thigh, or anterior thigh, or lateral thigh?”
ROB CATLIN, VP SALES & MARKETING at ENDO: “In our phase 2B study…we did study both buttocks and thighs.
“But for Phase 3, we focused just on buttocks. We did separate data generation to go for our approval. And when you get an FDA approval, it’s an incredibly heavy burden. So we bifurcated [divided into two branches or forks] buttocks and thighs and we focused on buttocks.
“That being said—and that’s what our indication is, is in buttocks—we’re continuing to do data generation for thigh, because I know that a lot of doctors are gonna have questions about how you treat this, so we’ll have the compliant resources if you need that information. But right now, we’re focused just on the buttocks. But we are doing data generation on the thighs.”
6 | The thighs require at least a different injection style
“We did a study about a year and a half ago looking at different injection techniques and it turned out that based on the study, the injection technique for the buttock and the injection technique for the thigh—it turned out to be different.
“So we’re still learning more and I think the more we get to market and the more we do that data generation, we’re gonna learn more how to treat other areas.” – Rob Catlin, VP Sales & Marketing at Endo
No, Men Can’t Be Treated With Qwo
That Qwo isn’t likely to work on men is a product of nature, not of modern fickle times or cancel culture. It has to do with “why cellulite affects 90% of all women but not many men.” The very structure and extrusion pattern of adipose tissue (fat) is different from men to women.
In women, the fibrous bands are arranged vertically, causing columns through which the fat can more easily protrude or herniate upward. In men, these fibrous bands are angled in a manner that makes cellulite less likely.
The Cost of Qwo is Unknown But is Likely to be Between $2,000 – $5,000
Endo has not yet released a cost of Qwo. By all current indications, it is likely to run between three and five thousand dollars. Qwo is also almost guaranteed to be priced per vial. No other pricing structure makes sense for an injectable. Thus, patients requiring treatment in a wider area or on more cellulite dimples will naturally need more sessions (and vials) to see full results and will thus end up paying more than patients with only minimal cellulite dimples.
“Somewhere in the range of $2,000 to $5,000” – Dr. Darren Smith
Board-certified plastic surgeon Dr. Darren Smith speculates “somewhere in the range of $2,000 to $5,000 seems to be a reasonable prediction. But again, that’s not based on any real data, that is purely speculation on my part based on what we’re seeing in the cosmetic injectable and cellulite treatment market right now.” There’s no telling if his 20% off non-surgical services discount will extend to Qwo.
“That is the million-dollar question… I really hope it will come and be reasonably priced.” – Dr. Bruce Katz.
In a July 2020 virtual presentation on Qwo, Dr. Bruce Katz was asked how much a procedure like this is likely to cost. “That is the million-dollar question,” said Dr. Katz. “Unfortunately we don’t know yet because the company hasn’t come out with the pricing.
“But I told them when we had a meeting in February, that this has to be priced moderately. It can’t be expensive because so many women have cellulite. Like I said, 80 to 90 percent of women have cellulite, we want this to have wide access. We want all kinds of women in all economic groups to be—for this to be accessible to them. So I hope, you know, I really tried to give them the message that this should be fairly priced and not ridiculously expensive.
“And, you know, that has happened unfortunately in other treatments. Kybella, you may be familiar with, which is a fat-melting chemical. That was priced way too high and as a result was not popular. The company didn’t do well with it. And, Celfina also, was priced way too high and has not been very popular. So, I really hope they will come out and be reasonably priced.” – Dr. Bruce Katz
Art of Skin MD in Solana Beach, CA is accepting $1,000 deposits for Qwo treatment
Dr. Melannie Palm served as a clinical investigator on the FDA trials. Art of Skin MD projects that Qwo may be available as soon as April 2021, and was briefly accepting $1,000 deposits for a Qwo treatment series. Deposits didn’t guarantee treatment at that rate, but was something of a promotional gesture—and an indication Qwo will likely cost well more than that.
Risks, Reactions & Side Effects

No Recovery Downtime Was Required In Qwo Clinical Trials
Expect to be able to return to almost all of your professional and personal activities immediately after treatment, barring those that would bare your bum to the world. If you’re planning Qwo to be ready for the summer beaches, do it months in advance.
Though no recovery or patient downtime was required, patients did experience mild to moderate soreness, swelling, and bruising and some used Tylenol or ibuprofen to deal with the discomfort.
Dr. Sachin Shridharani, MD and FACS at Luxurgery in New York participated in Qwo clinical trials. “Just expect some social downtime, maybe a little bit tender as well in the injection site, but nothing that my patients required anything more than some Tylenol or Ibuprofen to help alleviate some of those normal symptoms,” says Shridharani. “Now, the bruise can last a couple weeks so if you’re planning on doing this and going on your beach holiday, I would tell you either plan well ahead of time for me to help you get rid of that cellulite before you’re going to be in your bikini, or wait till afterwards”
Most Qwo Injection-site Pain Lasts 7 Days
For most patients, pain at the injection sites isn’t extreme. It’s easily bearable and usually requires no pain medication. Injection site pain was mostly self-resolving for patients and typically lasted no longer than seven days.
Most Qwo Injection-site Bruising Lasts 14 Days
Bruising is the most common and dramatic side effect of Quo and is at its worst immediately after the first Qwo treatment session. With each subsequent treatment, the severity and duration of bruising reduces greatly. Bruising from Qwo is usually self-resolving, requires no further treatment, and should typically last less than 14 days.
Most Qwo Side Effects Resolve Within 21 Days
In clinical trials for Qwo, adverse reactions lasted less than 21 days. “Generally, adverse reactions had a duration of less than 21 days,” says prescribing information.
The Most Common Side Effects of Qwo are Injection-site Pain, Bruising, Itching, and Redness
The following adverse reactions occurred in 1% or more of the Qwo trial participants:
- 84% experienced bruising at injection sites
- 48% had injection site pain
- 33% had nodules form at injection sites
- 15% had severe itching (pruritus)
- 9% had reddening of the skin (erythema)
- 8% had discoloration at injection sites
- 8% had swelling at injection sites
- 3% experienced warmth at injection sites
Comparing adverse reactions among those who received Qwo and those given a placebo:
| REACTION | QWO PATIENTS (424) | PLACEBO PATIENTS (419) | DIFFERENCE |
| Bruising | 84% | 21% | +63% |
| Pain | 48% | 10% | +38% |
| Nodule (Rounded lump) | 33% | 1% | +32% |
| Pruritus (Severe Itching) | 15% | 1% | +14% |
| Erythema (Skin Redness) | 9% | 5% | +4% |
| Discoloration | 8% | 1% | +7% |
| Swelling | 8% | 1% | +7% |
| Warmth | 3% | 0% | +3% |
About That Bruising: “You could be eggplant purple for a couple of weeks.”
Cue the “intense” and “impressive” bruising.
Bruising at injection sites is the most pronounced adverse resection. In clinical trials, 84% of Qwo recipients experienced it. As Qwo is individually injected in the divots and dimples of cellulite, bruising is also widespread.
“We tell patients you could be eggplant purple for up to a couple weeks after treatment,” says Dr. Palm.
The good news, however, is that while the bruising certainly looks dramatic, it doesn’t actually hurt and it resolves on its own.
“The first treatment is hallmarked by a pretty big bruise,” says Shridharani. “I often tell my patients, ‘Don’t be surprised if you look like you fell skiing or something like that.’ It’s not going to be as painful as that by any means, but you’re going to have a pretty sizable bruise. That’s a normal part of the process.”
“With each subsequent treatment cycle, the swelling and the bruising and a little bit of that downtime significantly started to decrease.
“We just gotta get you through that first treatment and then you’re going to do great.”
“So it’s not the stick. It’s not the needle.” – Dr. Grant Stevens
Note above that the patients injected with a placebo during clinical trials experienced 63 percent less bruising at injection sites, even though they were injected by a needle.
That’s because the bruising caused by Qwo isn’t solely caused by the needle but is thus far thought to be a byproduct of Qwo’s as-yet-unknown mechanism of action. Here’s how Endo’s VP Sales and Marketing describes it in the Technology of Beauty podcast episode mentioned earlier.
ROB CATLIN, VP SALES & MARKETING at ENDO: “The bruising is pretty pronounced. It’s demarcated and it’s, it’s impressive. We call it aubergine but it’s impressive bruising.
“And doctors said why is it bruising like that? So as part of that study I mentioned with Dr Sachin, we actually went in and we looked at what’s happening. And what we believe is happening is that once you inject—what’s important to note is that with Qwo, you’re not affecting any sort of arteries or veins because those are really protected by the Type IV collagen and with Qwo you’re targeting Type I to [and] III. But as you get to that sort of extracellular matrix, there’s these venules [very small veins] and what we believe is happening, is that they’re leaking.
“So that every time you inject, those venules are leaking so that’s why you’re seeing that, that bruising.”
DR. GRANT STEVENS: “So it’s not the stick. It’s not the needle. You think it’s the chemical causing leaky venules?”
ROB CATLIN, VP SALES & MARKETING at ENDO: “So that’s, you know, again, when you do a trial you don’t get to say what you think, you have to report what it is. And so it’s bruising. But based on the research and the science we’re continuing to investigate, that’s what we believe. So it feels almost like it’s part of the mechanism of action but that’s what we believe it might be.”
What’s In a Name?

Qwo is Manufactured by Endo Global Aesthetics Limited in Dublin, and Distributed by Endo Aesthetics LLC in Pennsylvania
Endo is an international pharmaceuticals manufacturer and publicly-traded company (ENDP) headquartered in Dublin, Ireland. US Corporate Offices are located in Malvern, PA. The company loosely traces its history back to 1920 to a family-run pharmaceutical company called Intravenous Products of America, Inc., that in 1935 changed its name to Endo Products. Today, Endo is a “specialty pharmaceutical company committed to helping everyone we serve live their best life through the delivery of quality, life-enhancing therapies.” Herein, let’s use Endo for the Irish manufacturer, and Endo LLC for the U.S. distributor.
Qwo is Pronounced Kwoe
Qwo is pronounced pretty much exactly how it looks.
But if that’s not obvious enough, rest assured that information from manufacturer Endo Global Aesthetics provided 19 March 2020 to the Center for Drug Evaluation and Research for its proprietary name review clarifies that the intended pronunciation of Qwo is indeed, “kwoe.”
Qwo, quo, kwoe.
The 4th Name Was a Charm
Before settling on the name “Qwo,” Endo submitted for consideration four different names to the Center for Drug Evaluation and Research.
Thanks to redactions, we don’t know what those names were, but we do know that CDER wasn’t in love with them and that the first of the four names was withdrawn by Endo.
“Thus, Endo Global Aesthetics Limited submitted the name, Qwo, for review on March 19, 2020.”
“Qwo” is an Evocative Brand Name That Blends the Quo in “Status Quo” with the Wo in “Women”
As to the definition and derivation of the name, the company provides neither. CDER notes that the proposed name houses two standard medical abbreviations—QW for once weekly, and WO for work order or written order.
But while a prescription is required for Qwo, Qwo isn’t administered once weekly. And there’s a much better explanation than the simplistic hidden abbreviations theory.
”Apparently it’s supposed to stand for ‘W-O’ for women, and Q, meaning that it is going to change the status quo for women,” says Katz.
Board-certified dermatologist and plastic surgeon Dr. Melanie Palm likewise served as a clinical investigator for Qwo. “Today, we are going to talk about not being status quo,” she says into the camera, 14 Jan 2021. “There’s a new kid on the block and it’s called Qwo.”
Qwo is short.
It rolls off the tongue.
And it rhymes with “woah.”
It’s an evocative brand name perfectly suited to a product that promises to be as game changing as it does.
San Diego based branding agency, Ignyte tells us such evocative names leave welcome room for interpretation and enable a brand to tell a powerful story, ultimately “creating a brand that’s bigger than just the products or services you offer.”
Kudos, Endo. You nailed it.
References:
1. FDA,Drug Approval Package, QWO 2. Patient Information Qwo 3. FDA, Highlights of Prescribing Information 4. https://www.qwo.com/ 5. https://www.qwo-hcp.com/ 6. FDA Drug Approvals and Databases 7. Dr. Whitney Bowe, “A dermatologist on why we get cellulite and the brand new treatments for it,” 16 July 2020 8. Dr. Timothy Jochen, What YOU Need to Know about QWO: Injectable Treatment for Cellulite, 25 February 2021 9. Dr. Grant Stevens, The Technology of Beauty Podcast, 1 December 2020 10. Pilot study of Dermal and Subcutaneous Fat Structures by MRI in Individuals that differ in Gender, BMI, and Cellulite Grading 11. QWO in New York City with Dr. Sachin Shridharani, 7 July 2020 12. Proprietary Name Review, Center for Drug Evaluation and Research (CDER), 12 June 2020 13. Endo Press Release: U.S. FDA Approves Qwo™ (collagenase clostridium histolyticum-aaes), the First Injectable Treatment for Cellulite 14. QWO in New York City with Dr. Shridharani, retrieved 13 March 2020 15. Modern Aesthetics, Endo’s Qwo Scores FDA Nod for Cellulite Treatment, 7 July 2020 16. Endo 17. Dermatology Times, First injectable for cellulite approved by FDA, July 8, 2020 18. Darren Smith, M.D., Qwo Cellulite Treatment 19. Cellulite Institute, Foods that cause cellulite, 17 October 2017 20. Drugs@FDA: FDA-Approved Drugs 21. FDA, BLA Approval Letter 22. FDA, Highlights Of Prescribing Information 23. CDER, Administrative and Correspondence Docs


